Analytical InstrumentationInstrumentation
What is pH meter ? How pH meter works?
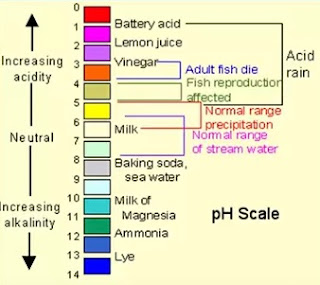
pH meter
A pH meter is an electronic device used for measuring pH(acidity or alkalinity) of a liquid.
A typical pH meter consist of a special measuring probe connected to an electronic meter that measures and displays the pH reading.
pH meter Probe
The probe is a key part of a pH meter,it is a rod like structure usually made up of glass.
At the bottom of the probe there is a bulb,the bulb is sensitive part of a probe that contains the sensor.
Never touch the bulb by hand and clean it with the help of an absorbent tissue paper with very soft hand,being careful not to rub the tissue against the glass bulb in order to avoid creating static.
To measure the pH of a solution the probe is dipped into the solution
The probe is fitted in an arm known as the probe arm.
How does a pH meter works?
A pH meters measures the concentration of the hydrogen ions [H+] in a solution
An acidic solution has far more positively charged hydrogen ions in it than an alkaline solution,so it has greater potential to produce an electric current under certain conditions
It is like a battery that can produce a greater voltage
A pH meter takes advantage of this and work like a typical voltmeter.
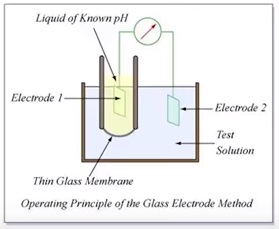
It consist of a pair of electrodes connected to a meter capable of measuring small voltages,on the order of mili volts.
It measures the voltage (electrical potential) produced by the solution whose acidity we are interested in compares it with the voltage of a known standard solution and uses the difference in voltage (the potential difference) between them to calculate the difference in pH.
pH Meter Calibration and Use
For very precise work the pH meter should be calibrated before each measurement.
Calibration should be performed with at least two standard buffer solutions that span the range of pH values to be measured.
For general purpose buffers at pH 4.01 and pH 10..0 are acceptable
For more precise measurements,a three buffer solution calibration is preferred
The calibration process correlates the voltage produced by the probe (approximately 0.06 volts per pH unit) with the pH scale.