Flame Ionization Detector
- The most accurate gas chromatographic detector for hydrocarbons like butane and hexane is the flame ionization detector (FID).
- In order to detect organic chemicals, flame ionization is the most reliable method.
- The FID evolved from the flame thermocouple detector in which a stream of hydrogen is burnt at a small jet over which a thermocouple is situated.
- Flame ionization, along with other detectors like thermal conductivity, thermionic or electrolytic conductivity, can be used to study biochemical compounds.
- There is often more carbon than any other element in biological molecules. Because of the higher carbon content and the sensitivity of flame ionization, it may be possible to detect a specific molecule with greater ease with flame ionization than with the other methods.
- The FID is the gas chromatographic detector for volatile hydrocarbons and numerous carbon-containing substances, with a linear range for 6 or 7 orders of magnitude (106 to 107) and the range lies in picogram or femtogram which is of low thereby limits the detection

Which form of elements/ chemical is responded by FID and what are special feature?
- Other than formic acid, responds to all organic substances.
- With hydrocarbons, the response is highest, and it falls with substitution. Does not react to inorganic compounds, with the exception of the vapor of Groups I and II elements.
- Due to the low noise level, sensitivity is high.
- The examination of aqueous extracts and studies on air pollution benefit from the absence of sensitivity to water, permanent gases, and inorganic chemicals.
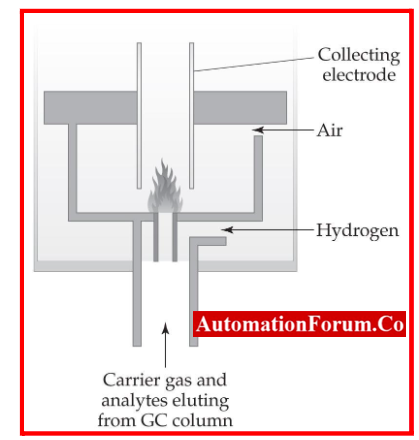
Construction and Working of Flame Ionization Detector
- Consists of an electrometer system in a separate unit next to the gas chromatograph and a stainless steel burner assembly mounted in the detector compartment.
- It is frequently put beside the thermal conductivity cell. As the column’s effluent enters the burner base, millipore filters remove contaminants.
- Hydrogen
- At the bottom of the jet, hydrogen is mixed with the gas stream, and air or oxygen is supplied axially around the jet. At the tip, which also serves as the cathode and is separated from the body by a ceramic seal, a hydrogen flame burns.
- Collector electrode
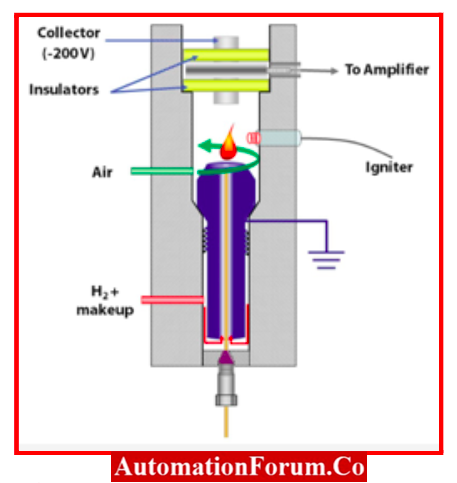
- Above the burner tip is a platinum collecting electrode. A FID consists of a collecting plate and an air/hydrogen flame.
- Effluent from the GC column travels through a flame, decomposing organic molecules and releasing ions in the process.
- The ions are collected by a biased electrode and then cause an electrical current to flow.
- The FID has an impressive dynamic range and is quite sensitive, but it has the unfortunate side effect of ruining the sample.
- Normally, FIDs are heated separately from the chromatographic oven. Heating is required to avoid condensation of water produced by the flame as well as any obstructions to solute flow from the column to the flame. The column end should be inserted into the jet once the flame has been extinguished, and it should then be softly held in place by loosening the connection.
- Make the column end into the detector jet gradually until it is about 1 to 2 mm below the jet tip. To keep the connection in place, tighten it. Capillary column connections shouldn’t be overtightened.

Mechanism
- Hydrogen and air are added to the column’s effluent before being electrically ignited at a tiny metal jet Ions and electrons produced by the majority of organic molecules can transmit electricity via the flame.
- To collect the ions produced by a hydrogen/air flame, there is an electrode placed above the flame.
- A signal is produced and amount of ion hitting the collector is counted.
- A range of resistors from 107 to 1010 ohms is connected in series with the flame gases.
- Using a vibrating reed electrometer, sensitivities up to 5 x 1013 Amps can be provided.
- An instrument for counting carbon that generates electricity in direct proportion to the number of ions or electrons generated in the flamed gases.
- To create charged species, the organic molecules go through a variety of processes including thermal fragmentation, chemi-ionization, ion molecule, and free radical reactions.
- The quantity of ions generated is roughly inversely proportionate to the number of molecules and reduced carbon atoms present in the flame.
- The flame ionization detector is a mass-sensitive device as opposed to a concentration-sensitive one since it responds to the quantity of carbon atoms entering the detector per unit of time.
- This detector benefits from the fact that variations in mobile phase flow rate have little impact on detector response.
Normal combustion.
Burning methane in air produces carbon dioxide and water vapor during normal combustion

During combustion a consistent amount (about 0.0002%) of the molecules in this process instead:(Simplified to Make Clear)
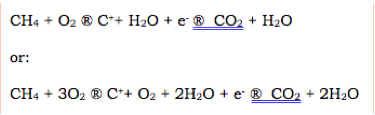
The FID can then find these intermediate, oppositely charged products:
Limitations
- This detector responds best to molecules that simply contain carbon and hydrogen, but it is less responsive to molecules that contain “heteroatoms” like oxygen.
- For instance, formaldehyde’s (CH2O) response to the FID is quite subpar compared to methane’s (CH4) excellent response. Therefore, it may be desirable to use a different detector in place of the FID to find highly oxygenated molecules or sulphides.
- Aldehydes and ketones can be analyzed with a photoionization detector and sulphides can be determined using a flame photometric detector, respectively.
Advantages of FID
- Simplicity, Dependability, Adaptability, and Simplicity of use.
- Since inorganics (He carrier gas) don’t react, the low background signal enables LOD that is 100–1000 times lower than TCD.
Disadvantage of FID
- FID cannot identify inorganic compounds.
- All compounds that pass through the FID flame oxidize.
- Remove sample
- Additional gases and controllers are needed.
Key Questions Flame Ionization Detector
What use does a flame ionization detector serve?
- It is used in science to analyze in a gas stream is called a flame ionization detector (FID).
- It is commonly employed in gas chromatography as a detector. This is a mass-sensitive instrument since it measures the number of ions per unit of time.
What is a flame ionization detector FID usually utilized to measure?
- Measure concentration of hydrocarbon
What is the mechanism of FID detector?
- In order to function, the FID relies on the detection of ions produced when organic molecules are burned in a hydrogen flame.
- The amount of organic species in the sample gas determines how many of these ions will be produced.
- These ions are detected by creating a potential difference between two electrodes.
Why does hydrogen used in FID?
Hydrogen is frequently utilized with capillary columns but is also employed as a carrier gas for packed columns. Hydrogen carrier provides more options for optimal linear velocities or flows, may be produced on demand from water with a suitable hydrogen generator, and costs less than helium.
What is the temperature of a FID detector?
A minimum temperature of around 20 to 50 degrees Celsius above the highest column temperature, up to the maximum allowed detector temperature, or a minimum temperature of 150 degrees Celsius for stable detector functioning, is used to calculate the suitable FID temperature.
What is the fuel gas in FID?
A gas that doesn’t ionize is necessary for FID. Therefore, inert gases are the perfect match. These gases, such as argon, helium, and nitrogen, have a low reactivity with other chemicals. In the past, helium has been the preferred carrier gas for FID.
What is the principle of flame ionization?
The preferred technique for determining the concentration of hydrocarbons (HC) in the automobile emissions business is the flame ionization detector (FID). The sample gas is fired up inside the FID’s hydrogen flame. Any hydrocarbons present in the sample will emit ions when burned.
What is the principle of the FID detector?
The FID works by detecting the ions that are produced when organic molecules burn in a hydrogen flame. The amount of organic species present in the sample gas stream directly relates to the formation of these ions.
What is the instrumentation of FID?
The components of a flame ionization detector (FID) are a collection plate and a hydrogen (H2)/air flame. Effluent from the GC column passes through a flame, decomposing organic molecules and producing ions along the way. The ions are captured by a biased electrode and used to create an electrical current.