- What is meant by Conductivity?
- How is conductivity measured?
- Units of Conductivity
- Conductivity Meter
- Conductivity Measurement principles
- Explain different types of Conductivity cells and compare it?
- Compare Advantages & Disadvantages of 2-pole and 4-pole cell?
- Applications of conductivity measurements
- How ph meter differs from conductivity?
- Factors affecting conductivity
What is meant by Conductivity?
- A solution, metal, gas, or, more broadly, any materials, have conductivity if it allows them to pass an electric current. Cations and anions carry current in solutions, whereas electrons carry it in metals.
- Solution’s conductivity measurements can reveal important details about a solution’s composition and nature of its constituent particles.
- A material’s conductivity determines its capacity to transmit or carry electricity or an electric current. It denotes the presence of charged ions or particles from dissolved electrolytes that make the solution conductive.
- The test solution has a high conductivity if the ion concentration is within permissible limits.
- The level of ionic strength in aqueous solutions ranges from ultra-pure water’s low conductivity to concentrated chemical samples’ high conductivity.
- Several variables affect how well a solution conducts electricity, including:
- Concentration
- Mobility of Ions
- Ion Valence
- Temperature of the solution
- It is advantageous for examining the characteristics of solutions, such as the total dissolved solids (TDS), for the evaluation of water quality.
- The meter also determines whether the solution is at the propertemperature and displays the results on the monitor.
- Electric current gearbox capacities might range. Electrolytes are substances having conductive solutions.
How is conductivity measured?
- Conductivity can be measured by applying an alternating electrical current (I) to two electrodes dipped in a solution and then observing the voltage that results (V). During this process, the anions go to the positive electrode while the cations move to the negative electrode, and the solution acts as an electrical conductor.

Units of Conductivity
- Siemens per centimeters (S/cm) are the units used to measure conductivity.
- Resistivity is used in place of conductivity in a few high purity water sectors, primarily the semiconductor and pharmaceutical ones. The opposite of conductivity is resistance. S/cm is equivalent to the earlier unit mho/cm., the conductivity unit can also be mho/cm
Conductivity Meter
- A conventional conductivity meter measures potential (V) by applying an alternating current (I) to two active electrodes at an ideal frequency
- The conductance (I/V) is computed using both the current and the voltage.
- The conductivity is then displayed by the conductivity meter using the conductance and cell constant.
Cell constant x conductance = Conductivity.
Note that the current source is changed to make the measured potential (V) and reference potential (Er) identical (about 200 mV apart).

Conductivity Measurement principles
- Conductive principle
- Inductive principle
Conductive principle
A cell having two metal or graphite electrodes in direct contact with the electrolyte solution is used to measure contact conductivity. The conductivity meter applies an AC current to the electrodes, and the conductance is calculated from the resulting AC current that flows between the electrodes. Pure water conductivity can be measured using this technique. Its primary flaw is that the cell is prone to corrosion and coating, which significantly lowers the reading. There may also be polarization effects in extremely conductive fluids, which cause measurements to be nonlinear.
- 2-electrode measuring cells
- 4-electrode measuring cells
2-electrode measuring cells
- The most straightforward conductivity measuring cell design is this one. For general industrial measurement, the 2-electrode measuring cell is more than sufficient.
- Two electrodes and a housing that holds the two electrodes together make up this cell. Between the two electrodes is applied a continuous AC voltage.
- The measurement signal is the current that is passing through the solution being tested.
- The cell constant and electrode surface characteristics for this kind of cell are determined by the intended usage of the cell.

- A large measurement signal is implied by a small cell constant. When conductivity values are low, this effect is desirable.
- The instrument may become overloaded at higher measured values. Thus, the range is the only factor that determines the ideal cell constant.
| Examples | Range of Conductivity | Cell Constant |
| Distillate, High purify, Condensate, Fully-desalinated | g< 10mS/cm | K’£0.1cm-1 |
| Drinking Water, Ground & Surface Water | g< 10-10,000mS/cm | K’£ 1 cm-1 |
| Sea & Saline Water | g>10mS/cm | K’£ 10 cm-1 |
4-electrode measuring cells
- Two pairs of electrodes are present in the measurement cells.
- One pair monitors the voltage applied across the measured solution, while the other pair measures the current.
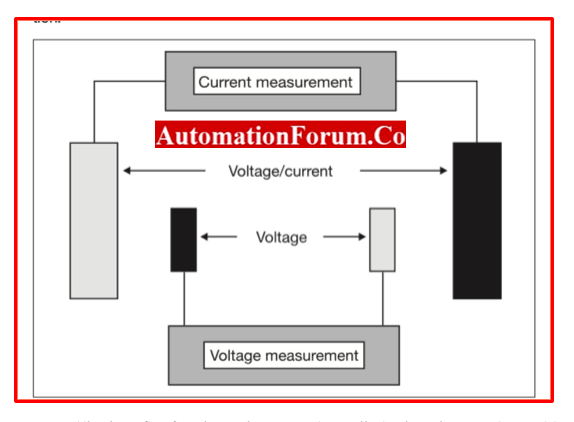
- The benefit of 4-electrode measuring cells is that they are insensitive to interfering resistances, such as those caused by impurities, long connecting cables, or polymerization.
- Due to these effects, the electrodes’ applied voltage to the solution being monitored is reduced, resulting in low values.
- The voltage across the measured solution is determined by the second set of electrodes. By using an electronic adjustment depending on the measured current/voltage values of the two electrode pairs, the instrument can accommodate for an interfering resistance.
- A layer of dirt or any extra resistance, for instance, constantly reduces the current and voltage by the same percentage.
- Within its limitations, the instrument may calculate the conductivity value as it measures both parameters.
Inductive measuring cell – Toroidal “Inductive” conductivity
- By running an AC current via a toroidal drive coil, which induces a current in the electrolyte solution, one can measure the toroidal conductivity (see image below).
- The pick-up toroid, a second toroidal coil, receives current from the generated solution current. The solution conductivity affects how much current is generated in the pick-up toroid.

- The fact that the toroidal coils are not in contact with the solution is the primary benefit of toroidal conductivity. They are either exterior to a flow through cell or enclosed in a polymeric substance.
- Disadvantages of toroidal conductivity measurements is they are less sensitive than contacting measurements.
- The toroidal solution current that it causes fills the area around the sensor, thus toroidal sensors are often larger than contacting sensors. Thus, a larger pipe is required for the installation of toroidal sensors.
Explain different types of Conductivity cells and compare it?
2-pole cell
In a conventional 2-pole cell, the voltage produced by applying an alternating current between the two poles is measured. Only measuring the solution resistance is the goal.

3-pole cell
The 3-pole cell, which was previously more prevalent, has been replaced by the 4-pole cell. The third pole, which was connected to pole 1, had the advantage of allowing the field lines to be guided and constrained in an ideal manner, limiting measurement dispersion and minimizing influences from the beaker’s volume and the location of the cell within it (the “field effect”) on the measurement. When determining the cell constant, it ensures greater reproducibility, leading to more reproducible results.

4-pole cell
- In a 4-pole cell, the 2 outer rings receive current in order to maintain a constant potential difference between 2 inner rings
- The two electrodes are not polarized since the voltage being measured is done with a very small current.
- The applied current will immediately correlate with the conductivity.
- Due to the measurement volume being precisely defined inside the tube, the geometry of 4-pole cells with an outer tube minimises the beaker field effect. Therefore, the measurement is unaffected by the location of the conductivity cell within the measuring vessel or the sample volume.

Flow-through cell
- These measurements can be carried out in a sealed liquid system that is airtight and also for small sample volume.
- A flow cell must be used if a measurement is to be made in only pure water. Air contact must be avoided. This occurs when the airborne carbon dioxide reacts with water to produce hydrogen carbonate ions, which changes the conductivity.
Two applications are possible for circulation cells:
- Circulation: Throughout the measurement, the solution flows continuously.
- Pipette: Draws a certain amount of solution into the cell. Small sample volumes are best suited for this technique.

Compare Advantages & Disadvantages of 2-pole and 4-pole cell?
2pole cell – Advantages
- Maintenance is easier
- Use with sample changer
- Economical
- Recommended for fluids or samples with suspension
2 pole cell – Disadvantages
- Field effects: the cell needs to be in the middle of the measuring device.
- Only cells that don’t span the plates
- Polarization in samples with high conductivity
- Using a reference with a value close to the measurement value, calibrate precise measurement for more than 20 years
4 pole cell – Advantages
- Over a very wide conductivity range
- Linear Measurement and calibration in various ranges
- Cells are of Flow through or submerged type
- Ideal for tests of high conductivity
- Using cell capacitance compensation low conductivity measurements are possible.
4 pole cell -Disadvantages
- Micro samples should not be used
- Immersion depth is of 3 to 4 cm
- Use with a sample changer is inappropriate
Applications of conductivity measurements
Measurements of conductivity are essential in many technological and environmental fields. Depending on the application, the measurement may occur in a lab, on location with a hand-held equipment, or continuously, for instance, in a process environment.
Consider the ensuing practical applications in greater depth. Measurement of conductivity in:
- Wastewater treatment facilities;
- Galvanizing operations;
- Beverage bottling operations;
- Pharmaceutical manufacturing operations;
- Power plants;
- Desalination facilities
How ph meter differs from conductivity?
| Conductivity Meter | ph Meter |
| Voltages are measured using a potentiometer instrument. | Potentiometer used to gauge the presence of hydrogen ions in a solution. |
| Comparing a known voltage to an unknown voltage in order to measure the voltage | Voltage between two electrodes is measured, and the result is shown as a pH value. |
| Voltage measurement units | Unit-less |
Factors affecting conductivity
The conductivity is affected by various factor
- Cell constant
- Contamination
- Movement of ions in the solution
- Geometry
- Concentration
- Polarization
- Cable resistance and capacitance
- Temperature of the solution
What is meant by TDS, TDS Factor & Salinity?
The TDS factor is used to multiply conductivity values by a known mathematical factor to produce TDS readings. The element depends on the sources used to develop the standard.
Salinity is an arbitrary unit-less quantity that represents the weight of dissolved salts in seawater.